Adding a third anti-malaria drug to current artemisinin-combination therapies (ACTs) provides effective treatment against multidrug-resistant falciparum malaria in Southeast Asia, say Mahidol and Oxford University researchers in a study published today in The Lancet.
The clinical study is the first to assess the efficacy, safety and tolerability of two triple artemisinin-combination treatments (TACTs) to treat falciparum malaria. Combining well-matched existing drugs provides mutual protection against resistance. This triple drug approach is used successfully in the treatment of tuberculosis and HIV infections, so using TACTs should extend the useful life of the few remaining effective and affordable antimalarial drugs, the study authors say.
“Triple artemisinin combination therapies (TACTs) were shown to be highly effective in parts of Cambodia, Vietnam and Thailand where most conventional ACTs no longer work. In these areas TACTs could be deployed soon to treat multidrug resistant malaria. Where artemisinin resistance or partner drug resistance has not yet emerged, deploying TACTs could delay the emergence and spread of antimalarial drug resistance,” said University of Oxford Professor Arjen Dondorp, principal investigator of the study and Deputy Director of the Bangkok-based Mahidol Oxford Tropical Medicine Research Unit (MORU).
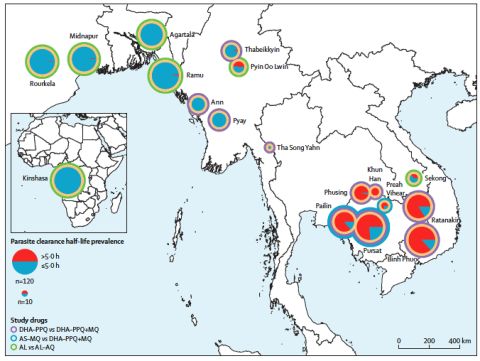
The Tracking Resistance to Artemisinin Collaboration (TRAC) II study is an open-label, randomized trial in 1,100 patients with uncomplicated falciparum malaria in 18 sites in 8 countries – Cambodia, Thailand, Myanmar, India, Laos, Vietnam, Bangladesh and the Democratic Republic of the Congo (DRC). It investigated two different TACTs, dihydroartemisinin-piperaquine+mefloquine and artemether-lumefantrine+amodiaquine. The study found that the new drug combinations were safe and well tolerated and were highly effective, also in infections where the current two-drug artemisinin combination therapies, ACTs, are failing because of multidrug resistance.
Global progress against malaria has stalled over the past four years. In 2018, there were an estimated 228 million malaria cases and 405,000 deaths (mostly in African children).
The Greater Mekong Subregion (GMS) in Southeast Asia is historically the origin of antimalarial drug resistance. In the past resistant parasites have spread from there to Africa, causing millions of deaths. In recent years resistance to artemisinins and their partner drugs in current ACTs have emerged and spread in the GMS. A repetition of the spread of antimalarial drug resistance from the GMS to Sub-Saharan Africa would increase both the number of malaria cases and deaths due to malaria. To avoid a disastrous repeat of history, countries in the GMS are aiming for malaria elimination, but success depends critically on the continued effectiveness of antimalarial drugs – and these are failing increasingly.
“We have an opportunity with TACTs to break the repeated historical cycle of waiting for antimalarial drug resistance to emerge and spread before changing therapy,” said study co-author University of Oxford Professor Sir Nicholas White. “There is a narrowing window of opportunity to prevent resistance derailing malaria control and making elimination impossible.”
Since TACTs use existing, affordable and generally well tolerated antimalarial drugs, they could be made widely available in the very near future. Using TACTs will buy important time before new antimalarial compounds become available and should prolong the life of the existing antimalarial drugs.
“Widescale deployment of TACTs could delay or prevent the emergence and spread of antimalarial resistance in areas not yet affected by antimalarial drug resistance. It could also prevent the importation of antimalarial drug resistance from the GMS to the Indian subcontinent and Sub-Saharan Africa, which would put millions of lives at risk,” said TRAC II coordinator and article lead author Dr Rob van der Pluijm from MORU.
MORU has begun DeTACT, a large 5-year follow-up project funded by DfID, to evaluate TACTs and have them ready for use as front-line drugs to fight malaria across Africa and Asia by the project’s end.
“Our new Development of Triple Artemisinin Combination Therapies (DeTACT) project is working with pharmaceutical companies to develop TACTs into a deployable product, using these products in placebo-controlled trials in Asia and Africa, conducting TACT-related mathematical modelling, market positioning and bioethics studies, to provide evidence and strategies necessary to rapidly transition from ACTs to TACTs as frontline treatment for uncomplicated falciparum malaria, worldwide,” said MORU’s Dr Chanaki Amaratunga, DeTACT Coordinator and TRAC II study co-author.
Funders
The TRAC II study was funded with support from the United Kingdom Department for International Development, UK (DfID), with additional funding support from the Wellcome Trust, UK; Bill & 78 Melinda Gates Foundation, USA; Medical Research Council, UK; and the National Institute of Health, USA.
References
Triple Artemisinin based Combination Therapies (TACTs) versus ACTs for uncomplicated Plasmodium falciparum malaria: a multi-centre, open label, randomized clinical trial by the Tracking Resistance to Artemisinin Collaboration. Rob van der Pluijm, et al. The Lancet, Published online March 11, 2020 https://doi.org/10.1016/ S0140-6736(20)30552-3
Commentary
Are three drugs for malaria better than two? Philip J Rosenthal (UCSF), Comment, The Lancet, Published online March 11, 2020 https://doi.org/10.1016/ S0140-6736(20)30560-2
Background
Determinants of dihydroartemisinin-piperaquine treatment failure in falciparum malaria in Cambodia, Thailand and Vietnam: a prospective clinical, pharmacological and genetic study. Rob van der Pluijm et al.(2019). The Lancet Infectious Diseases, 22 July 2019. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(19)30391-3/fulltext
Evolution and expansion of multidrug resistant malaria in Southeast Asia: a genomic epidemiology study. William Hamilton and Roberto Amato et al. (2019). The Lancet Infectious Diseases, 22 July 2019. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(19)30392-5/fulltext
Spread of artemisinin resistance in Plasmodium falciparum Malaria. Ashley EA et al. N Engl J Med. 2014 Jul 31;371(5):411-23. doi: 10.1056/NEJMoa1314981.
Notes for editors
The Mahidol Oxford Tropical Medicine Research Unit (MORU), www.tropmedres.ac, @MORUBKK, is a research collaboration between Mahidol University (Thailand) and University of Oxford and Wellcome (UK).


 ©
MORU 2020. Photographer: Mehul Dhorda
©
MORU 2020. Photographer: Mehul Dhorda